What Is The Formal Charge On Phosphorus In A Lewis Structure
The formal charge is 0. Chemistry questions and answers.
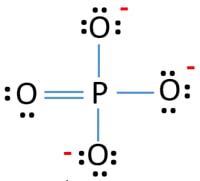
Po43 Lewis Structure Phosphate Ion
In the lewis structure of PO 43- three is a double bond between phosphorous atom and one oxygen atom.

What is the formal charge on phosphorus in a lewis structure. This is how we calculate the formal charge. A 1 b -2 c. What is the formal charge on phosphorus in POCIZF if we construct its Lewis structure without violating the octet rule.
If you check the formal charges for POCl 3 youll find that you need a double bond between the Phosphorous and Oxygen atom in order to have the formal charges equal zero. It is a member of phosphoranes a phosphorus hydride and a mononuclear parent hydride. You might think youve got the correct Lewis structure for Cl 3 PO at first.
For Br the formal charge 7 052 6 0 holds true for all the three bromine atoms So now we put the single bonds to get our perfect Lewis Structure. 2 CO D-2 El. Between other oxygen atoms there are only single bonds with phosphorous atom.
For the ammonium ion NH 4 each H is still 0. Phosphorane is a phosphorus hydride consisting of a single pentavalent phosphorus carrying five hydrogens. Five chlorine atoms will make five single bonds with five electrons of the phosphorus.
What is the formal charge of phosphorus in the proper Lewis structure of phosphate ion. Our tutors have indicated that to solve this problem you will need to apply the Lewis Dot Structure. What is the formal charge on phosphorus in a Lewis structure for the phosphate ion that satisfies the octet rule.
For P the formal charge 5 056 2 0. 5 - 4 1 so N has a 1 charge. Remember Phosphorous is below Period 2 on teh Periodic.
Formal charge V - N - B2 where V Numb View the full answer Transcribed image text. For each H atom it has 1 bond and thus 1 electron so its formal charge is also 0. Also each oxygen atom has a -1 charge.
In this way the octet of phosphorus will be completed and three lone pairs will be left on each chlorine. The parent hydride of the phosphorane class. This is good because all the formal charges of each atom must add up to the total charge on the molecule or ion.
Lewis structure of PO 43- ion. Now N has 4 bonds and no lone pairs so it owns 4 electrons. If you need more Lewis Dot Structure.
5-7-2 4-40 6-51 5-6-1 4-40 6-60 5-50 4-40 Remember a charge on the molecule means you need to add an electron when counting your valence electrons. The Lewis structure for Cl 3 PO requires you to place Phosphorous P in the center of the structure since it is the most electronegative. In the POCl 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center.
What is the formal charge of the phosphorus atom in the following Lewis structure. In the Lewis structure for POCl 3 there are a total of 32 valence electrons. Formal Charge practice problems.
Formal Charge practice you can also practice Lewis Dot Structure. Always count your valence electrons -1N-CO -1NCO -1NC Oxygen gets the -1 charge because it is the most electronegative atomSolution 6-7 -1 O. Assign the labels O1 O4 starting from the top oxygen and going clockwise.
Then O2 4 are identical in formal charge as they are equivalent oxygens in this representation. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry in a biological context sulfur almost always follows the same bondingformal charge pattern as oxygen while phosphorus is present in the form of phosphate ion PO 4 3 where it has five bonds almost always to oxygen no lone pairs and a formal charge of zero. What is the formal charge on phosphorus in a Lewis structure for the phosphate ion PO4 that satisfies the octet rule.
3- 0. -3 FREE Expert Solution Show answer. Phosphate is actually PO3 4 and has the following resonance structure 3 other permutations.
An other atoms are bonded to P A 3 B 5 C 1 D 0 E l. Get the detailed answer.
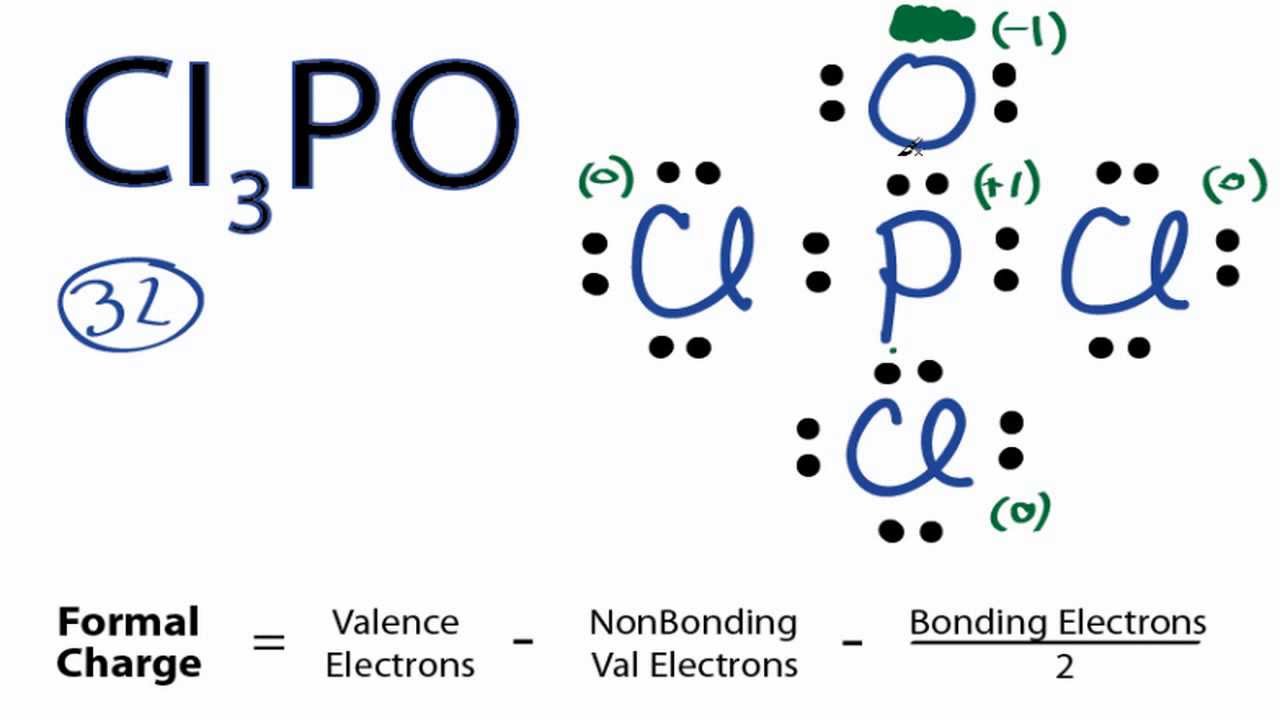
Cl3po Lewis Structure How To Draw The Lewis Structure For Cl3po Youtube

Pcl3 Lewis Structure Phosphorus Trichloride Youtube

What Is The Formal Charge On Phosphorus In A Lewis Structure For The Phosphate Ion That Satisfies Th Youtube

Po4 3 Lewis Structure The Phosphate Ion Youtube

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube

What Is The Formal Charge Of Phosphorus In Clutch Prep

How To Draw The Lewis Dot Structure For Pi5 Phosphorous Pentaiodide Youtube

Post a Comment for "What Is The Formal Charge On Phosphorus In A Lewis Structure"