What Is The Lewis Structure Of Cif3
The actual structure of ClF3 with slightly bent angles. This liquid is used in electroplating mining and as a precursor for several compounds.
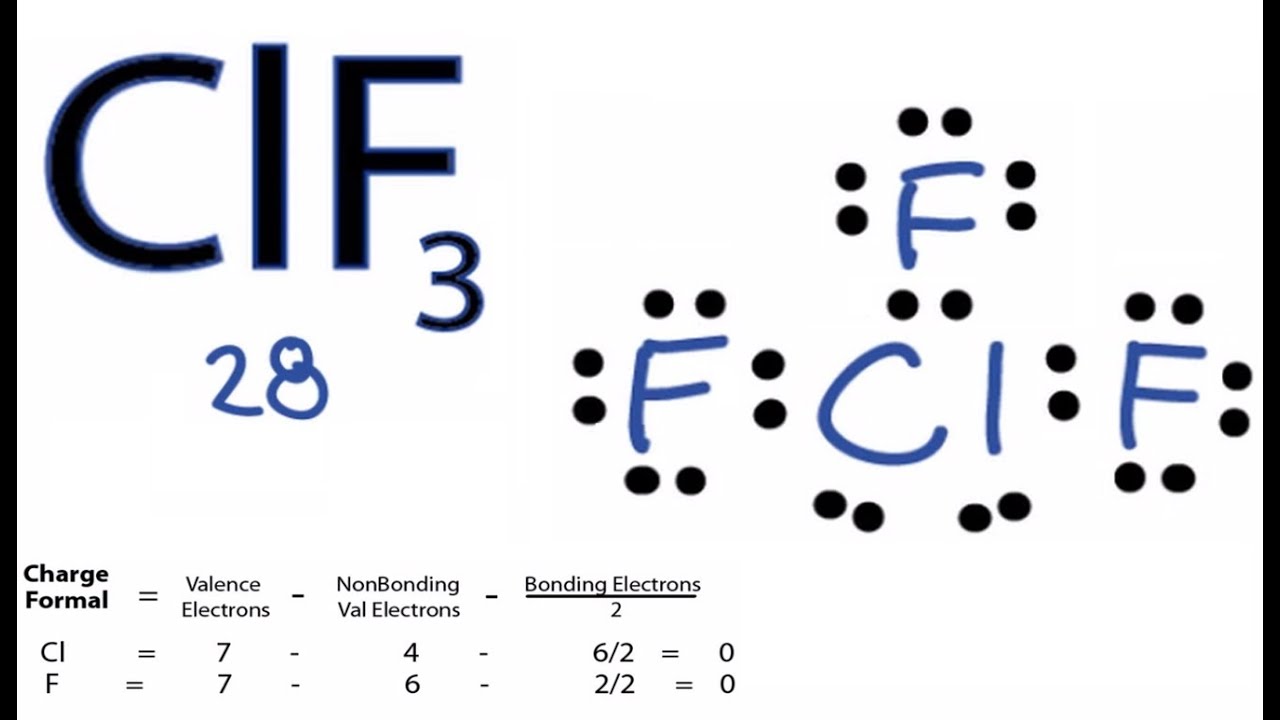
Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube
Alternatively a dot method can be used to draw the ClF 3 Lewis structure.

What is the lewis structure of cif3. Is the molecule polar why. Draw the Lewis structure for ClF3. By signing up youll get thousands of step-by-step solutions to your homework questions.
What is the molecular geometry. ClF3 has 5 electron groups around the central Cl atom. Lets see how to draw lewis dot structure for NF3 with easy steps.
The Lewis Structure of CIF3 is pictured below. The molecular geometry of ClF 3 is approximately T-shaped with one short bond 1598 and two long bonds 1698 . The result is a T-shaped molecule.
You should be able to see this when you draw the Lewis structure of the molecule. Hydrogen Cyanide is a colorless flammable and poisonous chemical liquid. Include a 3-D drawing and dipole arrows in your answer.
Chlorine has seven valence electrons and each fluorine atom has one valence electron. With 5 electron groups around the central atom the molecule will adopt a trigonal bipyrimid shape. This structure is very similar to NCl3 and NH3.
Represented by the chemical formula HCN is one of those molecules that has an interesting Lewis structure. Contact with organic materials may result in spontaneous ignition. Lewis structure of ClF3 Expert Answer 100 19 ratings Previous question Next question.
8 rows ClF3 lewis dot structure. Put chlorine in center and arrange fluorine atoms on the sides. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs.
The lewis structure of chlorine trifluoride is. When we talk about the hybridization of chlorine trifluoride we have to consider its central atom which is Cl. HCN Lewis Structure Molecular Geometry Shape and Polarity.
O EDG trigonal bipyramidal MG T-shaped O EDG tetrahedral MGtrigonal pyramidal O EDG trigonal bipyramidal MG trigonal bipyramidal O EDG trigonal planar MGbent O EDG pointy MG bendy. The two lone pairs take equatorial positions because they demand more space than the bonds. This atom contains 7 valence electrons while ClF3 should consist of 3 bond-pairs and 2 lone-pairs.
Lewis Structure Of ClF3. What type of intermolecular force will dictate the physical properties of this molecule. Calculate the total valence electrons in the molecule.
Lewis Structure Of ClF3. Lewis structure of ClF3. Simple steps for drawing the Lewis dot structure.
What is the electron domain geometry EDG and molecular geometry MG of this molecule. After using all valence electrons Look at the above ClF3 lewis. ClF3 Lewis Structure Molecular Geometry Hybridization and Polarity Chlorine trifluoride or ClF3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds.
This structure agrees with the prediction of VSEPR theory which predicts lone pairs of electrons as occupying two equatorial positions of a hypothetic trigonal bipyramid. The T-shaped structure of ClF3 showing the lone pair orbitals. If we take a closer look at the valence electronic configuration of Cl it is represented as 3s 2 3px 2 3py 2 3pz 1 3d.
NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds. Molecular formula CIF3 Draw Lewis structure for CIF3 What is the electron geometry. Because repulsions involving lone pairs are stronger than those involving bond pairs the F-Cl-F angle is a little under 180 so the molecule has a slightly bent T-shape.
An interhalogen compound having both Cl and F it has a density of around 379 gl and a molar mass of 9245 gmol. Three bonding and two nonbonding. This problem has been solved.
It reacts with water to form chlorine and hydrofluoric acid with release of heat. Arrange electrons until both nitrogen and fluorine get 8. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor.
These are arranged in a trigonal bipyramidal shape with a 175 F axial-Cl-F axial bond angle. So the total number of valence electrons is 10.

Clf3 Lewis And 3 D Structure Dr Sundin Uw Platteville

Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube
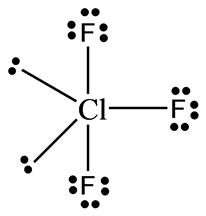
Solved Draw The Lewis Structure For Clf3 What Are Its Electron P Chegg Com

Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube

Clf3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Clf3 Lewis Structure Molecular Geometry Shape Is It Polar Or Non Polar

5 Draw The Best Lewis Structure For The Following Molecul Clutch Prep

Clf3 Lewis Structure Molecular Geometry Shape Is It Polar Or Non Polar

7 Draw Lewis Dot Structures For The Followingmolecules Cif3 Brainly In
Post a Comment for "What Is The Lewis Structure Of Cif3"