Why Does Bf3 Have A Trigonal Planar Shape
Isnt it true that in order to be trigonal planar there should be 4 bonded pairs and 0 lone pairs. Boron has only 3 valence electrons so when it bonds with F there will only be 3 electron pairs around the boron atom.
Why Does A Bf3 Molecule Have A Planar Shape While An Nh3 Molecule Is Pyramidal Quora
Thus BF3 is planar triangular.

Why does bf3 have a trigonal planar shape. Each atom bonded to Phosphorous provides 1 electron there are 3 Hydrogens so 1x33. The geometry of the BF3 is trigonal planar which helps to cancel out the dipole moments of the three BF bonds and at last the result of the dipole moments becomes zero. Repulsion theory predicts that these three e- pairs should find themselves at the vertices of an equilateral triangle bond angles of 120 degrees.
There are 4 electron pairs but from the molecule we can see there are 3 bonding pairs so there must be 1 lone pair. - no lone pairs. Thus three Fluorine atoms will occupy three corners of triangular planar structure.
In boron trifluoride the central boron atom undergoes sp 2 hybridization in order to account for the trigonal planar shape. The four centers of electrons around the B. - 3 bonded pairs.
B is at the center of the equilateral triangle formed by the F atoms. B contains three valence electrons 5B 23 It does not have lone pair of electrons. Question 10 1 point What is the shape of BF3 and why does it assume this shape.
While in BF3 B is surround by three F- atoms. So its shape is pyramidal. Isnt it true that in order to be trigonal planar there should be 4 bonded pairs and 0 lone pairs.
This also accounts for the. Trigonal pyramidal Because III. See full answer below.
If central atoms contains 5 bond repulsion units and if it doesnt contain lone pair on central atom the shape of the molecule is trigonal bipyramidal. - repel each other equally at 120 degrees. - the molecules are all aligned in one plane - essentially the molecule is flat.
Its a 4 marker asking explain why a BF3 molecule has the shape you have drawn Please help. The shape of the orbitals is planar triangular. The bond angles are compressed relative tothose in a perfect trigonal bipyramid due to lone.
It is a flat molecule with B surrounded by the F atoms in a trigonal planar arrangement of atoms. This means the shape is trigonal pyramidal. There exists repulsion force between lone pair and bond pair of electrons in NF3 molecule because of which it acquires pyramidal shape.
So its shape is trigonal planar. Since there is an atom at the end of each orbital the shape of the molecule is also planar triangular. BF3 is sp2 hybridized with no lone pairs.
Recommend 0 Comment 0. Boron trifluoride or BF3 is a nonpolar molecule because in BF3 fluorine is more electronegative than boron. If two bonds of trigonal biyramidal basic geometry are changed into two lone pairstherefore its molecular shape is.
ClF3 shows Trigonal bipyramidal geometry. - lone pairs repel more strongly than bonded pairs. The geometry of molecule of BF3 is Trigonal Planar With the reference of Chemistry Trigonal Planar is a model with three atoms around one atom in the middle.
Boron trifluoride or BF3 is nonpolar because the shape of the BF3 is highly symmetric. The three centers of electrons around the B repel and point toward the corners of a triangle. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle.
Probably marks for saying. This molecule is made up of 3 equally spaced sp 2 hybrid orbitals arranged at 120 o angles. Why does NO3- have a molecular geometry shape of trigonal planar.
BrF3 contains three bonded and two nonbonded electron domains giving a trigonal pyramidal e- domain geometry and a T shaped molecular geometry. The moment of the. Where as in BF3 the central atom Boron undergoes SP2 hybridization and has no lone pair of electrons.
The average F-B-F bond angle is 120 degrees.
What Is The Bond Angle Of A Trigonal Planar Molecule Such As Boron Trifluoride Bf3 Quora
Explain Why The Bond Angles Of A Trigonal Pyramidal Molecule Are Smaller Than Those Of A Trigonal Planar Molecule Quora
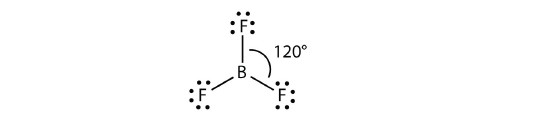
9 6 Molecular Shapes Chemistry Libretexts
Why Does A Bf3 Molecule Have A Planar Shape While An Nh3 Molecule Is Pyramidal Quora

Trigonal Planar Structure Examples Video Lesson Transcript Study Com
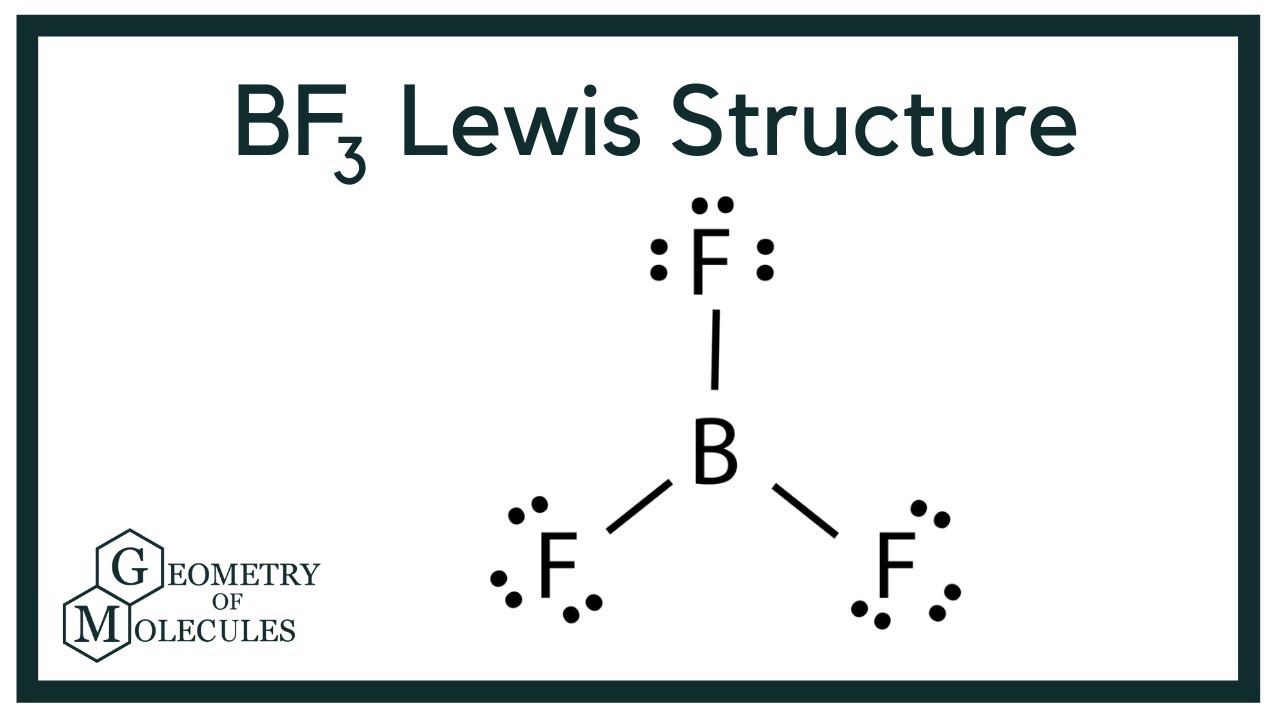
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
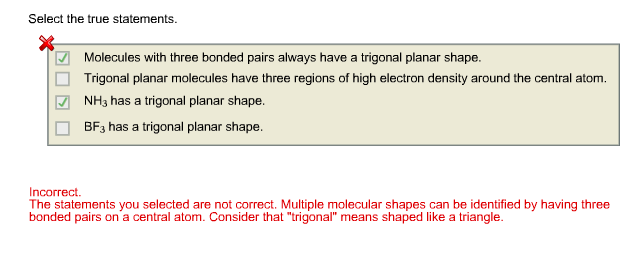
Select The True Statements Molecules With Three Chegg Com

Why Does A Bf3 Molecule Have A Planar Shape While An Nh3 Molecule Is Pyramidal Quora
Post a Comment for "Why Does Bf3 Have A Trigonal Planar Shape"