Shape Of Bcl3 Using Vsepr Theory
Electron pairs gives base shape Octahedral VSEPR base shape for 6 e-pairs Refcode. X B and Q H for gaseous boron hydride BH3.

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
The VSEPR theory argument gives the shape TRIGONAL PLANAR.
Shape of bcl3 using vsepr theory. The theory was first presented by Sidgwick and Powell in 1940. Boron forms three sp-p bonds with three chlorine atoms by using its half filled sp 2 hybrid orbitals. And both having a central atom from the.
ANumber of central atoms. The central atom B has only three bond pairs and no lone pair. The central atoms As has five bond pairs and no lon pair.
The central atoms As has five bond pairs and no lon pair. For each multiple bond doubletriple bond subtract one electron from the final total. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes.
The central atom has no lone pair and there are three bond pairs. Hence the shape is tetrahedral. Each chlorine atom uses its half filled p-orbital for the -bond formation.
Use the VSEPR model to predict the molecular geometry of a O. XNumber of surrounding atoms. Hence the shape is tetrahedral.
We are given the molecular formulas of a molecule and a polyatomic ion both conforming to the general formula AB. A linear B trigonal planar C bent D tetrahedral E trigonal pyramidal. BCl3 takes the shape of trigonal planar.
The central atom B has only three bond pairs and no lone pair. So the overall total number of electrons should be 2 this is the electron region number. Hence shape is triangular planar.
Hence it is trigonal planar. E Number of lone pairs on central atom. Hence the shape is trigonal bipyramidal.
What is the molecular shape of BCl3 as predicted by the VSEPR theory. Hence the shape is trigonal bipyramidal. The central atom Si has four bond pairs and no lone pair.
Apply VSEPR notation A X E. Discuss the shape of the following molecules based on VSEPR theory. Identify the central atom 2.
The CO 2 molecule has 2 double bonds so minus 2 electrons from the final total. Asked Jan 24 2020 in Chemistry by Nishu03 642k points chemical bonding. But the results of the VSEPR theory can be used to predict the positions of the nuclei in these molecules which can be tested experimentally.
We can see from the chart that BCl3 is an AX3 type molecule. Hence shape is linear. Hence shape is triangular planar.
The initial VSEPR shape for the CO 2 molecule is Tetrahedral. Valence Shell Electron Pair Repulsion Theory VSEPR is used to determine the shape and bond angle of a molecule. When lone pair electrons are present the parent structure are used as a guideline for determining the shape.
Lone pair electrons are also taken into account. If we focus on the positions of the nuclei in ammonia we predict that the NH 3 molecule should have a shape best described as trigonal pyramidal with the. Discuss the shape of the molecules using the VSEPR model.
Q-X-Q bond angle exactly 120o. However the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds which cannot be predicted using the Lewis electron-pair approach. Add one electron for each bonding atom 4.
Hence it is of the type EX3. BeCl2 has minimum energy when it is a linear molecule. The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule.
Add or subtract electrons for charge see Top Tip 5. Use VSEPR table to find the shape. For the above molecule VSEPR notation will be AX 3 E 0.
Boron in Group 313 has three outer valence electrons each of which pairs up with a hydrogen electron - dot and cross diagram on the right. The central atom Si has four bond pairs and no lone pair. Sample Exercise 91 Using the VSEPR Model.
Use lewis structure guidelines to draw the lewis structure of BCl 3. Block of the periodic table. The shape according to VSEPR theory is given below MoleculeNumber of electron pairs around central atomMolecular geometryBond anglesBeC l2 2Linear180oBC l3 3trigonal planar120oS iC l4 4tetrahedral1095oAsF 5 5trigonal bipyramidalthree 120o two 90oH 2 S6non linearbent92oP H 3 5trigonal pyramidal935o.
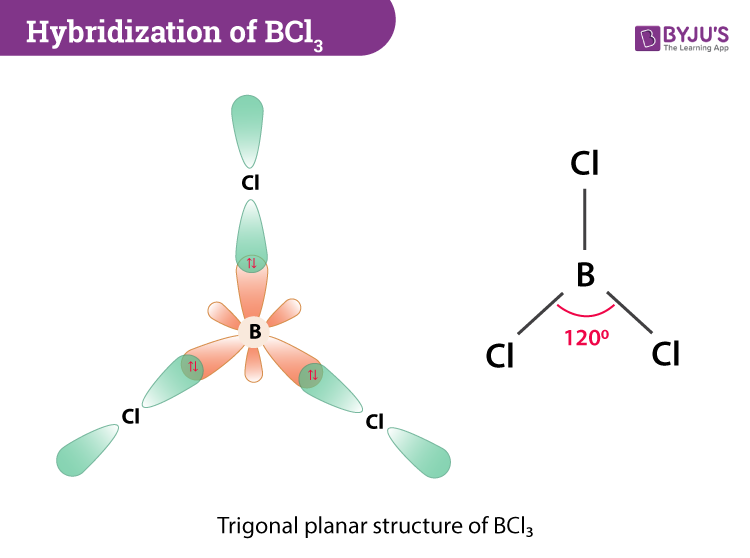
According to VSEPR theory the molecular geometry of boron trichloride is trigonal planar with a bond angle of 120 degrees. Count its valence electrons 3. Therefore the shape of the molecules are arranged so that the energy is minimized.
Thus the shape of BCl 3 is trigonal planar with bond angles equal to 120 o. The VSEPR chart is attached below. Asked Nov 28 2020 in Chemistry by Maisa 457k points chemical bonding.
AX 3 has trigonal planar shape. The VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell. Hence shape is linear.
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
What Is The Molecular Geometry Of Bcl3 Quora
Https Www Topperlearning Com Answer Explain The Shape Of Bcl3 Using Vsepr Theory Fk7v3yww

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
Https Www Topperlearning Com Answer Explain The Shape Of Bcl3 Using Vsepr Theory Fk7v3yww

How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube

Post a Comment for "Shape Of Bcl3 Using Vsepr Theory"